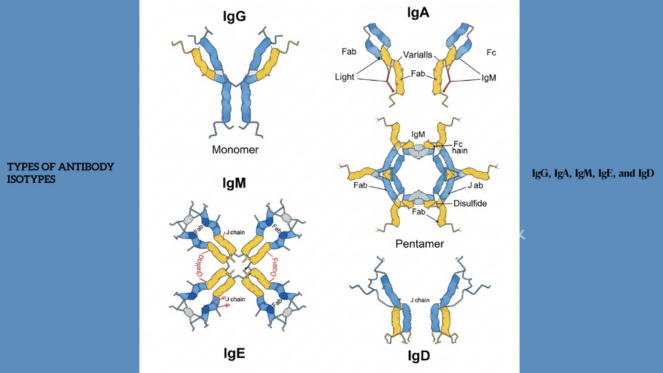
All Types of Antibody Isotypes Explained: IgG, IgA, IgM, IgE, and IgD
In this Article
All of the products listed in AAA Biotech’s catalog are strictly for research-use only (RUO).
Key Takeaways
- IgG is the most abundant, long-lasting antibody isotype. Also, it is able to cross the placenta for facilitating fetal immunity.
- IgA dominates in mucosal secretions, protecting entry points like the gut and lungs.
- IgM is the first responder in infections, forming pentamers for strong complement activation.
- IgE is vital for allergy responses and defense against helminths, despite low serum levels.
- IgD functions mainly as a receptor on immature B cells and aids respiratory immunity.
- Each isotype has a unique structure, distribution, and half-life.
- Specific subclasses (IgG1–4, IgA1/2) refine functions further.
- Isotype diversity ensures balanced systemic and mucosal immunity.
Immunoglobulins (Ig), known more commonly as “antibodies“, are glycoproteins that are produced by the body’s B lymphocytes and plasma cells as part of the body’s “adaptive immune system”. They are mainly needed to identify and neutralize antigens, be it pathogens such as viruses/bacteria, allergens, or toxins.
All antibodies have a common structural backbone, but vary in terms of the kind of heavy chain constant region that defines the specific isotype.
Human beings have five main isotypes of antibodies: IgG, IgA, IgM, IgE, and IgD. Although all perform the task of antigen recognition, each of the isotypes has specific structural aspects, patterns of distribution, half-lives, interaction with receptors, and immune activity.
We will break each isotype down into specific details, focusing on structure, function, location, and immunological significance.
Immunoglobulin: An Overview
An antibody is comprised of two light chains and two heavy chains, all of which are identical, and forms a Y-shape. The top tips of the Y has variable regions, which are the parts that bind to/recognize antigens, and the constant regions, which make the lower “stem” of the Y and determine the isotype of the antibody.
Key Parameters Differentiating Isotypes
| Isotype | Heavy Chain Type | Serum % | Average Half-Life | Primary Location | Notable Function |
|---|---|---|---|---|---|
| IgG | γ (gamma) | ~75% | 21 days (IgG1, IgG4), ~7 days (IgG3) | Serum, extracellular fluids | Secondary immune response, opsonization, placental transfer |
| IgA | α (alpha) | ~15% | 6 days | Mucosal secretions, breast milk, saliva | Mucosal defense, neutralizing pathogens at entry points |
| IgM | μ (mu) | ~10% | 5 days | Blood, initially released in the lymphoid tissues | First antibody produced, complement activation |
| IgE | ε (epsilon) | <0.01% | 2 days | Bound to mast cells and basophils | Allergy, parasitic infection immunity |
| IgD | δ (delta) | <1% | 3 days | Surface of immature B cells, respiratory tract | Acts as a B-cell receptor and modulates activation |
IgG: The Workhorse of The Immune System
The predominant antibody in serum and in extracellular fluid is IgG. It
constitutes approximately 75% of all immunoglobulins in the body at any one
time.
Structural Features
- Monomeric with two antigen-binding sites.
- Four subclasses: IgG1, IgG2, IgG3, IgG4. All of them differ
in the length of the “hinge” region and their capacity to induce complement
system or bind Fc receptors.
- The longest hinge region is IgG3, which also has the shortest half-life (approximately 7 days).
Major Functions
- Dominance of secondary response: IgG is produced in huge
amounts when the body is exposed to the same antigen in subsequent
exposures.
- Opsonization: Attaches Fc receptors to phagocytes in order
to encourage ingestion.
- Complement activation: IgG1 and IgG3 in particular activate
the classical complement pathway.
- Antiviral and antibacterial activity: Neutralizes toxins
and prevents pathogen adherence.
- Placental transport: Passive immunity of the baby only via its ability to cross the placenta (through neonatal Fc receptor, FcRn).
Subclass Specialization
| Subclass | Serum Proportion | Half-Life | Complement Activation | Role |
|---|---|---|---|---|
| IgG1 | ~65% of IgG | 21 days | Strong | Response to protein antigens |
| IgG2 | ~23% | 21 days | Weak | Response to polysaccharide antigens (e.g., bacterial capsules) |
| IgG3 | ~7% | ~7 days | Very strong | Excellent complement activator |
| IgG4 | ~4% | 21 days | None | Involved in chronic exposure, allergen tolerance |
IgA: The Mucosal Guardian
IgA constitutes approximately 15% of serum antibodies; however, more
significantly, it is the predominant immunoglobulin in the body’s mucosal
secretions.
Structural Features
- Exists in monomeric (serum IgA) and dimeric (secretory IgA, sIgA)
forms.
- The secretory component (SC) shields IgA against enzymatic destruction in
severe mucosal environments.
- Two subclasses: IgA1 (dominant in serum) and IgA2 (dominant in secretions like intestine, saliva).
Critical Functions
- Mucosal defense: Neutralizes pathogens at entry sites (gut,
respiratory tract, urogenital tract).
- Non-inflammatory response: Does not cause severe
stimulation of complement; does not cause much damage to tissues in the
mucosa.
- Immune exclusion: Prevents microbial adhesion to epithelial
cells.
- Neonatal immunity: Acquired through the transfer of breast milk to infants, which coats the gut surfaces in infants.
IgM: The First Responder
Approximately 10% of total serum antibodies is composed of IgM, but it is
actually the first antibody isotype that is produced upon exposure to the
antigen.
Structural Features
- Exists as a pentamer in circulation, with 10 potential antigen-binding
sites.
- J-chain links monomers in a pentameric structure.
- IgM is limited to the intravascular compartment largely by its large size.
Major Functions
- Primary response preeminence: First antibody isotype
produced upon infection.
- Firm complement activation: Excellent triggering of the
classical pathway, which is made possible by the pentameric structure.
- Agglutination: Valency is high, so the pathogens can be
properly “clumped”.
- B cell receptor (BCR): IgM is membrane-bound and monomeric before the class switching stage of immature B cells.
IgE: The Allergy and Parasite Mediator
The least prevalent is IgE (<0.01% of serum immunoglobulins, approximately 50-200 ng/mL), but disproportionately important in allergic disease and parasite resistance.
Structural Features
- Monomeric structure
- Mast cells and basophils have high-affinity Fc epsilon RI receptor binding, resulting in prolonged tissue half-life (although plasma half-life is relatively short, ~2 days).
Critical Functions
- Allergic reaction: Binding of the allergen to IgE on the
mast cell causes cross-linking of IgE, releasing histamine, leukotrienes, and
cytokines
- Helminth defense: Eosinophils express Fc eRII and are involved in the assault of IgE-coated helminths.
- Immediate immune signal: Activates the immune system toward antigens in the environment.
IgD: The B Cell Identity Marker
IgD is 1% of the serum immunoglobulins but is a critical B cell receptor (BCR) in early B cell differentiation.
Structural Features
- Monomeric with a δ heavy chain.
- Serum level: ~ <0.5 mg/dL, extremely low with respect to IgG or IgA.
Major Functions
- B cell activation: Co-expressed with IgM on immature B cell surfaces, which assists in the initiation of the signals that promote clonal selection.
- Respiratory immunity: Occurs in the upper airways mucosa and is used in local defense.
- Regulatory role: Has a role in preventing overactivation by harmless antigens in the environment.
Comparative Snapshot of All Isotypes
| Feature | IgG | IgA | IgM | IgE | IgD |
|---|---|---|---|---|---|
| Basic structure | Monomer | Monomer / dimer | Pentamer (serum), monomer (BCR) | Monomer | Monomer |
| Serum Level | Highest | Moderate | Moderate | Trace | Very low |
| Half-life (avg) | 21 days | 6 days | 5 days | 2 days | 3 days |
| Major site | Blood, fluids | Mucosal secretions | Blood, lymph nodes | Mast cells, basophils | B cells, respiratory mucosa |
| Placental transfer | Yes | No | No | No | No |
| Complement activation | Yes (IgG1, IgG3) | Weak (IgA1 only) | Very strong | No | No |
| Primary functions | Opsonization, neutralization, memory | Mucosal barrier, immune exclusion | First infection response, agglutination | Allergy, parasite immunity | B-cell activation marker |
Conclusion
The presence of the different antibody isotypes illustrate the different approaches of the immune system to protect against pathogens, control reactions, and tolerance.
IgG, for example, provides long-term systemic protection and immunity either post-infection or post-vaccination, while IgA is indispensable for its protection of mucosal surfaces, the very points of entry for most pathogens. IgM responds to an early antigenic challenge with a rapid mobilization, and IgE is the two-sided responder in defending against parasites and causing allergy.
Last but not least, IgD is low in serum, but it plays a critical role in determining the identity of B cells and initial immune control. The identification of the biological niche and the capabilities of each isotype is at the heart of immunology, diagnostics, vaccine design, and therapeutic intervention in immune-related disease.
Faq's
Why does IgG have the longest half-life compared to other antibody isotypes?
IgG attaches to the neonatal Fc receptor (FcRn), which preserves it by preventing lysosomal degradation and recycles it into circulation, increasing its half-life to 21 days.
What is the difference between IgA1 and IgA2?
IgA1 is the most common in serum, and IgA2 is in the mucosal secretions because it is more resistant to gut bacterial proteases.
Why is the level of IgE so low in circulation?
The Fc exchange of most IgE is fixed to FcεRI of mast cells and basophils. The level of free plasma IgE is low, as it is its primary reservoir being bound.
What are the uses of IgM in diagnostic tests?
IgM presence indicates a recent or acute infection since it is produced first during the primary immune response. Serological diagnostics is regularly performed using the detection of pathogen-specific IgM (e.g., anti-HAV IgM).
Does IgD have any role beyond being a B-cell receptor?
Yes, other than acting on naive B cells, IgD also plays a role in the protection of the respiratory mucosa and in the regulation of B cell tolerance to commensal or inert antigens.