Highest grade reagents available anywhere in the world.
AAA BioTech catalog carries only the finest quality reagents.
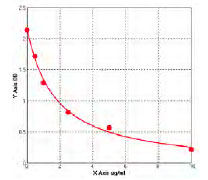
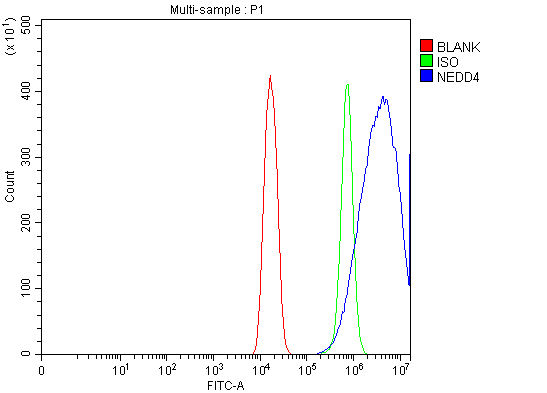
Highly validated and characterized monoclonal/polyclonal antibodies and recombinant proteins
The majority of AAA Biotech’s antibodies are highly validated and can be use in multiple applications such as ELISA, FC, ICC, IF, IHC, IP, WB, etc. We have antibodies available for rare species, in multiple conjugated forms or recombinant antibodies.
As for our high quality proteins, the majority have 90% purity, detected by SDS-PAGE while some are available in different tags such as Flag, GST, His, MBP, etc. We also carry high quality native and biologically active proteins.
AAA Biotech is constantly working to expand our capacity to provide recombinant proteins and antibodies to most target proteins.
Shop MAbs Shop PAbs Shop Proteins